异烟棒曲霉素C
外观
| 异烟棒曲霉素C | |
|---|---|
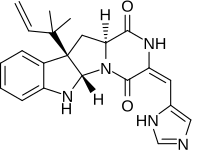
| |
| IUPAC名 (3E,5aS,10bR,11aS)-3-[(1H-Imidazol-5-yl)methylidene]-10b-(2-methylbut-3-en-2-yl)-6,10b,11,11a-tetrahydro-2H-pyrazino[1′,2′:1,5]pyrrolo[2,3-b]indole-1,4(3H,5aH)-dione | |
| 识别 | |
| CAS号 | 58735-64-1 |
| PubChem | 21608802 |
| ChemSpider | 10246629 |
| SMILES |
|
| InChI |
|
| InChIKey | SPWSUFUPTSJWNG-JJUKSXGLBA |
| 性质 | |
| 化学式 | C22H23N5O2 |
| 摩尔质量 | 389.5 g·mol⁻¹ |
| 外观 | 白色或灰白色固态 |
| 溶解性(水) | 溶于乙醇、甲醇、DMF、DMSO |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
异烟棒曲霉素C(英语:Roquefortine C)是一种真菌毒素,是一种天然2,5-哌嗪二酮衍生物[1]。其由不同真菌产生,特别是青霉菌菌种[2]。其最初从罗克福青霉菌中提取得到,这种菌种在商业上常被用作蓝纹奶酪、洛克福奶酪、丹麦蓝奶酪、斯蒂尔顿奶酪和戈贡佐拉奶酪成熟过程中的蛋白水解酶和脂肪分解酶的来源。
异烟棒曲霉素C是一种环状二肽,从哌嗪二酮环(色氨酸-脱氢组氨酸)二肽结构母体衍生得到,是几种青霉菌中常见的代谢产物。其被视为饮料、啤酒、红酒、肉、奶酪和面包中一种真菌污染物[3]。高浓度的异烟棒曲霉素C被归类为有毒化合物[4],特别是其具有强神经毒性[5][6]。意大利研究学者发在意大利本土奶酪中一般含有0.05至1.47 mg/kg异烟棒曲霉素C,但认为该含量范围“对消费者是安全的”[7]。研究人员通过研究其与哺乳动物的细胞色素P450酶的相互作用,其毒性机理和代谢过程得以知晓[4]。此外,异烟棒曲霉素C还对革兰氏阳性菌展现出抑菌活性[8],但仅限于含有血红素蛋白的菌类[4][9]。
异烟棒曲霉素C含有不常见的E-脱氢组氨酸残基,这使得其在酸性、碱性以及光照条件下容易异构化成其异构体异异烟棒曲霉素C(isoroquefortine C),即连接在2,5-哌嗪二酮环上的双键从E构型变成Z构型[10]。

其异构体不同于天然产生的异烟棒曲霉素C,在自然界中并不存在,且不能与铁离子结合。但两者均可以通过人工合成得到[10]。
相关条目
[编辑]参考文献
[编辑]- ^ Borthwick AD. 2,5-Diketopiperazines: Synthesis, Reactions, Medicinal Chemistry, and Bioactive Natural Products. Chemical Reviews. 2012, 112 (7): 3641–3716. PMID 22575049. doi:10.1021/cr200398y.
- ^ Kokkonen M, Jestoi M, Rizzo A. The effect of substrate on mycotoxin production of selected Penicillium strains. International Journal of Food Microbiology. 2005, 99 (2): 207–14. PMID 15734568. doi:10.1016/j.ijfoodmicro.2004.08.014.
- ^ Borthwick AD, Da Costa NC. 2,5-Diketopiperazines in Food and Beverages: Taste and Bioactivity. Critical Reviews in Food Science and Nutrition. 2017, 57 (4): 718–742. PMID 25629623. S2CID 1334464. doi:10.1080/10408398.2014.911142.
- ^ 4.0 4.1 4.2 Aninat C, Hayashi Y, André F, Delaforge M. Molecular requirements for inhibition of cytochrome P450 activities by roquefortine. Chemical Research in Toxicology. July 2001, 14 (9): 1259–1265. PMID 11559041. doi:10.1021/tx015512l.
- ^ SCBT. Roquefortine - A potent neurotoxin produced most notably by Penicillium species. [2024-07-25]. (原始内容存档于2016-03-16).
- ^ EPA. Penicillium roqueforti Final Risk Assessment. [2024-07-25]. (原始内容存档于2015-09-24).
- ^ Finoli C, Vecchio A, Galli A, Dragoni I. Roquefortine C occurrence in blue cheese.. J. Food Prot. February 2001, 64 (2): 246–51. PMID 11271775. doi:10.4315/0362-028x-64.2.246
 .
.
- ^ Kopp-Holtwiesche B, Rehm HJ. Antimicrobial action of roquefortine. Journal of Environmental Pathology, Toxicology and Oncology. December 1989, 10 (1–2): 41–44. PMID 2231314.
- ^ Aninat C, Andre F, Delaforge M. Oxidative metabolism by P450 and function coupling to efflux systems: modulation of mycotoxin toxicity. Food Additives and Contaminants. April 2005, 22 (4): 361–368. PMID 16019806. S2CID 9880652. doi:10.1080/02652030500073287.
- ^ 10.0 10.1 Shangguan N, Hehre WJ, Ohlinger WS, Beavers MP, Joullie MM. The total synthesis of roquefortine C and a rationale for the thermodynamic stability of isoroquefortine C over roquefortine C. Journal of the American Chemical Society. April 2008, 130 (19): 6281–6287. PMID 18412344. doi:10.1021/ja800067q.
