GS-6620
外觀
 | |
| 臨床資料 | |
|---|---|
| 商品名 | GS-6620 |
| 法律規範狀態 | |
| 法律規範 |
|
| 識別資訊 | |
| |
| CAS號 | 1350735-70-4 |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| 化學資訊 | |
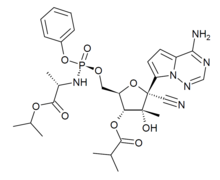
| 化學式 | C29H37N6O9P |
| 摩爾質量 | 644.6 |
| 3D模型(JSmol) | |
| |
| |
GS-6620是一種核苷酸類的抗病毒藥物,被開發用於治療丙型肝炎。儘管它在早期測試中顯示出有效的抗病毒作用[1][2],但由於它在腸道中的吸收率低且效果不穩定(無法預測血液中的濃度),未能成功製成口服劑。[3][4]目前進行的研究主要針對其它疾病(如伊波拉出血熱)[5][6]的治療。
參考文獻
[編輯]- ^ Cho A, Zhang L, Xu J, Lee R, Butler T, Metobo S, et al. Discovery of the first C-nucleoside HCV polymerase inhibitor (GS-6620) with demonstrated antiviral response in HCV infected patients. Journal of Medicinal Chemistry. March 2014, 57 (5): 1812–25. PMID 23547794. doi:10.1021/jm400201a.
- ^ Feng JY, Cheng G, Perry J, Barauskas O, Xu Y, Fenaux M, et al. Inhibition of hepatitis C virus replication by GS-6620, a potent C-nucleoside monophosphate prodrug. Antimicrobial Agents and Chemotherapy. 2014, 58 (4): 1930–42. PMC 4023746
 . PMID 24419349. doi:10.1128/AAC.02351-13.
. PMID 24419349. doi:10.1128/AAC.02351-13.
- ^ Murakami E, Wang T, Babusis D, Lepist EI, Sauer D, Park Y, et al. Metabolism and pharmacokinetics of the anti-hepatitis C virus nucleotide prodrug GS-6620. Antimicrobial Agents and Chemotherapy. 2014, 58 (4): 1943–51. PMC 4023801
 . PMID 24419340. doi:10.1128/AAC.02350-13.
. PMID 24419340. doi:10.1128/AAC.02350-13.
- ^ Gentile I, Coppola N, Buonomo AR, Zappulo E, Borgia G. Investigational nucleoside and nucleotide polymerase inhibitors and their use in treating hepatitis C virus. Expert Opinion on Investigational Drugs. September 2014, 23 (9): 1211–23. PMID 24848437. doi:10.1517/13543784.2014.921680.
- ^ De Clercq E. C-Nucleosides To Be Revisited. Journal of Medicinal Chemistry. March 2016, 59 (6): 2301–11. PMID 26513594. doi:10.1021/acs.jmedchem.5b01157.
- ^ De Clercq E. New Nucleoside Analogues for the Treatment of Hemorrhagic Fever Virus Infections. Chemistry, an Asian Journal. November 2019, 14 (22): 3962–3968. PMID 31389664. doi:10.1002/asia.201900841.
| ||||||||||||||||||||||||||
